Screen Bottom Boards (SBB) have a number of uses in our bee stewardship and are widely substituted for a solid bottom board. A 1939 Delaware beekeeper removed his solid bottom and left his colony open at the bottom, which he termed “bottomless” beekeeping [There truly is nothing “new” in our beekeeping practices]. Although many beekeepers use SBB to control varroa, BIP and PNW surveys point out they are not a highly effective varroa mite control tool.
Our PNW Honey Bee Survey asked respond-ents about screen bottom board use. Among Oregon and Washington hobbyist (backyarders or small-scale beekeepers) in the 2014-15 survey year, 79% of the 250 respondents said they used screened bottoms (66% used them on all their hives with the remaining 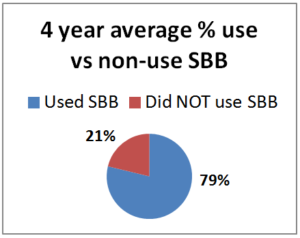 13% using them on a percentage of their hives). In 2015-16 period, 140 individuals used SBB on all and 41 individuals used on some of their colonies (74.4%) while 62 individuals DID NOT use SBB 25.5%). For 2016-17, 178 individuals used them on all their colonies and 46 did on some for a total use of 83%; 17% (47 individuals) DID NOT use them. For the most recent year 214 individuals used SBB on all their colonies, 40 on some =80% while 63 individuals did not use SBB 20%. The numbers have been consistent each year with range of use on all or some of hives from 74.4 to 83%.. The graph shows 4 year average of number of individuals who used SBB (79%) vs those NOT USING SBB (21%).
13% using them on a percentage of their hives). In 2015-16 period, 140 individuals used SBB on all and 41 individuals used on some of their colonies (74.4%) while 62 individuals DID NOT use SBB 25.5%). For 2016-17, 178 individuals used them on all their colonies and 46 did on some for a total use of 83%; 17% (47 individuals) DID NOT use them. For the most recent year 214 individuals used SBB on all their colonies, 40 on some =80% while 63 individuals did not use SBB 20%. The numbers have been consistent each year with range of use on all or some of hives from 74.4 to 83%.. The graph shows 4 year average of number of individuals who used SBB (79%) vs those NOT USING SBB (21%).
For each year comparing overwinter loss percentage of individuals using SBB on some or all of their hives with the smaller percent that did not use SBB revealed a small advantage to SBB use with slightly lower losses. In the 2014-15 season, there was a small difference of 2 percentage points for individuals NOT using SBB (27% loss rate) to those who used SBB (25%) on some or all of their colonies. For 2015-16 there was a marked difference in favor of those who used SBB; the 17% who did NOT use SBB had overall loss average of 48% compared to 55.5% overall loss average of the 83% of individuals who used SBB on all or some of their hives. The difference was even larger in 2016-17 but opposite; for the 13.6 that did NOT use SBB, their overall average loss was 59.4% compared to the 46% loss rate of the 86.3% who used SBB on some or all of the hives. In the
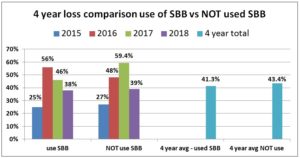
2017-18 season, the loss rate of those using SBB on all or some of their hives was 38% vs a 39% loss rate form those 63 individuals not using SBB. In three of four years there was a slight advantage to use of SBB with 1, 2 and 13.4% greater survival rate. The four year average – 41.3% loss level of those using SBB and 43.4% for those not using SBB (a 5% gain) illustrates how they are very minor in improving losses overwinter survival.
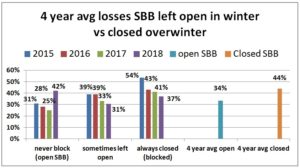
We also asked whether they blocked colony SBBs during the winter. IN 2014-15, the majority (51%) left them open over the winter period (never response), 19% sometimes blocked them and 31% said they closed them (always response) during the winter. For the next two seasons the majority response was to leave the screens open at the bottom of the colony overwinter. 2015-16 56% left open vs 26.5% closed and 2016-17 50% left open vs 31% closed. This past season 39% left them open, 17% sometimes did so and 44% closed them. When we examined winter losses for this response, there was an improvement in loss when the SBB were closed (24% vs 31% in 2014-15 for example, 39 vs 32% in 2015-16, 53.5% vs 36.6% in 2016-17 and 42% vs 37% this past winter.) Comparing the always blocked the over winter response with the never response and sometimes (left open) results in a 10 percentage point difference in favor of closing the SBB over the winter period.
With our Bee Informed National survey (www.beeinformed.org), use of SBB did not improve winter survival rate in any of our survey years when we directly compared their use with loss rates. However in survey year 2013-2014, northern beekeepers did have about a 10% decrease in losses when SBB were used compared to southern beekeepers. Experimental studies on SBB and mite population levels show either no or this slight, ~10%, improvement in reducing mite population levels when SBB are used.

So what can SBB do to benefit our bees?
A Screen bottom board is a multifunctional IPM tool. Using a screen bottom aids greatly in hive ventilation. It can be used with upper entrances at the covers or with ventilation ports added to hive bodies. Air circulation for better ventilation can be further aided by pushing the outer frames inward a bit to allow air circulation upward between the outer frame and box wall. Heavy burr and brace combs, sometimes due to incorrect bee spacing between the hive boxes, when both the bottom bar and the dropping of the top bars below the rim creates a ½ inch of larger space, and heavy propolis use in the fall, may reduce good air circulation in the boxes.
During winter, beekeepers at more northerly locations or higher elevations often close or reduce the screen opening beneath their colonies. This is thought to e useful to have the queen use more of the lower box for egg laying. Since clusters are in the top box in early spring and European bees are slower to expand downward more slowly this potential negative can be easily managed by reversal of brood boxes in the mid-spring. It is not advisable with an open screen however to have wind blowing into and through the bottom of a winter colony. An air space beneath the colony, with screen bottom board left open, is not detrimental to colony wintering. The dead air space and, moderating soil temperatures may be helpful. Often this dead space beneath a screened bottom can be created with hive stand configuration.
Another advantage of a screen bottom board is that it provides for a convenient garbage pit to remove debris and fallen mites from a colony. The original Langstroth hive had such a feature but it was discontinued when the hive construction was simplified. They were thought to promote wax moth with the design Langstroth used.
The greatest benefit of a SBB is as a mite monitoring tool. It is a key element in knowing HOW MANY mites are present since we know that most likely mites are already in the colony, There are some SBB that make this monitoring, getting our mite number assessment, easier than others. SBB with mite monitoring boards are the most useful. You can make your own or purchase such boards. Designs that allow you to insert the monitoring board with minimal disturbance are best. However you can make your own and use petroleum jelly to trap fallen mites and a wide putty knife to clean it for reuse for very little expense.
Reading a mite monitoring board beneath a colony can be difficult. Mites are tiny and hard to see (use of pow-power magnifier and strong light can help focus the board assessment. Leaving a monitoring board underneath a colony for a single day or 2-3 at most will help reduce debris drop. If bees are cleaning out old pollen, are uncapping brood or honey cells or are heavily bringing in nectar or building new comb can result in higher levels of debris. Ants may enter and feed on the mites. seeds from grasses or nearby plants that blow into the hive and land on the screen board can greatly confuse the counting.
Screen Bottoms may offer some improvement for some beekeepers, particularly where winter confinement period is long and when mite populations are lower. In is not clear if this improvement is due to mites alone or to the other effects a screen vs closed bottom may have on colony survivability (see below). As regards varroa mites they should be considered a tool that may reduce winter losses when used in combination with other mite control treatments and tools.
May 2018 Dewey M. Caron
View PDF of this report here

 About a dozen brave individuals gathered at the Zenger apiary colonies Sunday April 15th, during a steady Oregon liquid “sunshine” rain for dead colony forensics with Dewey. Photo right by Mandy Shaw.
About a dozen brave individuals gathered at the Zenger apiary colonies Sunday April 15th, during a steady Oregon liquid “sunshine” rain for dead colony forensics with Dewey. Photo right by Mandy Shaw. The first dead-out we looked at (photo above) proved to be a tough diagnosis.
The first dead-out we looked at (photo above) proved to be a tough diagnosis. Photo of the three frames with brood shows the frame with a patch of compact brood (held in my right hand) and two frames with very scattered brood (one in my left hand and the third on top of adjacent hive; this frame is shown isolated in photo right). Full super on ground. Photos by Deb Caron.
Photo of the three frames with brood shows the frame with a patch of compact brood (held in my right hand) and two frames with very scattered brood (one in my left hand and the third on top of adjacent hive; this frame is shown isolated in photo right). Full super on ground. Photos by Deb Caron. The second dead-out was a more standard necropsy. Hive was a spring split,that struggled all season. It had 2 shallows. Colony had a 19 mite count (6%+) in September and was treated with 2 formic pads between the two boxes. It was alive in March (this spring) but noted as small. It was fed dry sugar on paper (some still remaining) and provided with a frame of sugar candy.
The second dead-out was a more standard necropsy. Hive was a spring split,that struggled all season. It had 2 shallows. Colony had a 19 mite count (6%+) in September and was treated with 2 formic pads between the two boxes. It was alive in March (this spring) but noted as small. It was fed dry sugar on paper (some still remaining) and provided with a frame of sugar candy. All frames, except one with high number of drone cells, could be reused for anew colony installation (package, swarm, split). Brush off dead cluster and from bottom board. If inclined wash mold with bleach or vinegar solution.
All frames, except one with high number of drone cells, could be reused for anew colony installation (package, swarm, split). Brush off dead cluster and from bottom board. If inclined wash mold with bleach or vinegar solution.